Pooling samples for sequencing does not appreciably improve the measured batch effect
Benjamin R Babcock
Pooled_Sequencing.RmdDifferent Sequencing Pools (“sequenced separately”)
Processing the Seurat Objects 4-B & 5-A
Data filtering & normalization
library(BatchNorm)
# Import unfiltered Seurat PBMC4 object & extract "4B" (included with 'BatchNorm' package)
data(package = 'BatchNorm', PBMC4)
Idents(PBMC4) <- PBMC4[["orig.ident"]]
PBMC4B <- subset(PBMC4, idents = "Sample_4B")
# Import unfiltered Seurat PBMC5 object & extract "5A" (included with 'BatchNorm' package)
data(package = 'BatchNorm', PBMC5)
Idents(PBMC5) <- PBMC5[["orig.ident"]]
PBMC5A <- subset(PBMC5, idents = "Sample_5A")
# Merge "sequenced together" samples into a single object and remove unnecessry objects from the workspace
PBMCs <- merge(PBMC4B, y = PBMC5A)
rm(list = c('PBMC4', 'PBMC5', 'PBMC4A', 'PBMC5A'))
# Run "standard" Seurat workflow:
# Including filtering by mitochondrial percentage (+5 SD)
# Including data normalization, variable gene selection and gene scaling (performed on all samples together)
PBMCs <- PBMCs %>%
MitoFilter() %>%
NormalizeData(normalization.method = "LogNormalize", assay = "RNA", scale.factor = 10000) %>%
NormalizeData(verbose = FALSE, assay = "ADT", normalization.method = "CLR") %>%
FindVariableFeatures(selection.method = "vst", nfeatures = 2000) %>%
ScaleData() %>%
RunPCA(npcs = 30)Selecting the appropriate number of Principal Components for UMAP reduction
## Identify correct numbers of PCs
## (Takes up to 5 minutes. Not run while rendering vignette for time)
# PBMCs.pca.test <- TestPCA(PBMCs)
# PBMCs.pca.test[, 1:20]
## 15 PCs with z > 1
## Proceed with 15 PCs for dimensional reduction & clustering
## Visualize PCs plotted by standard deviation:
ElbowPlot(PBMCs)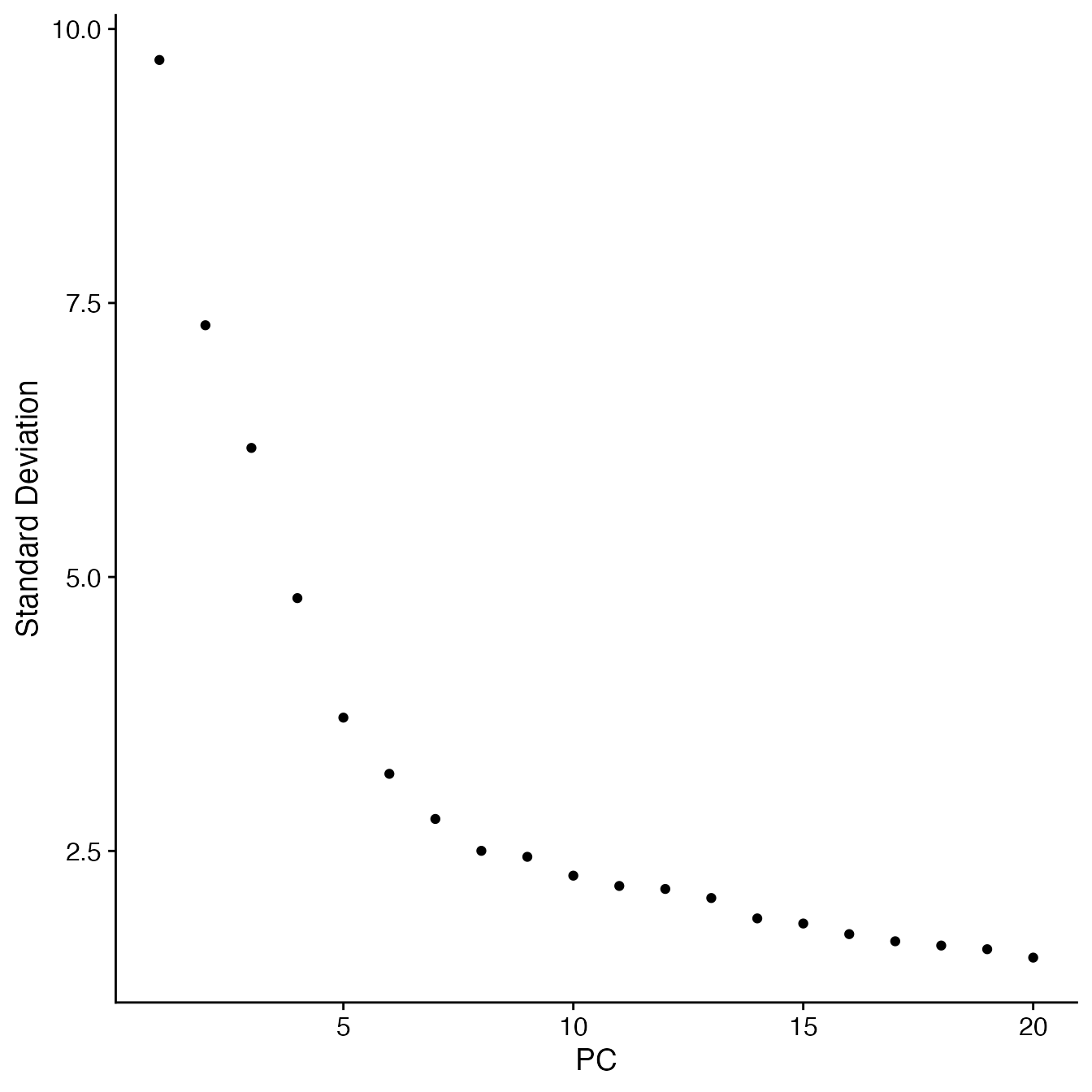
Generating a UMAP and clusters
PBMCs <- PBMCs %>%
RunUMAP(reduction = "pca", dims = 1:15) %>%
FindNeighbors(reduction = "pca", dims = 1:15) %>%
FindClusters(resolution = .8)## Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
##
## Number of nodes: 10128
## Number of edges: 354085
##
## Running Louvain algorithm...
## Maximum modularity in 10 random starts: 0.8781
## Number of communities: 19
## Elapsed time: 1 seconds
UMAPPlot(PBMCs, cols = colors.use, group.by = "orig.ident") + ggtitle("Default Workflow")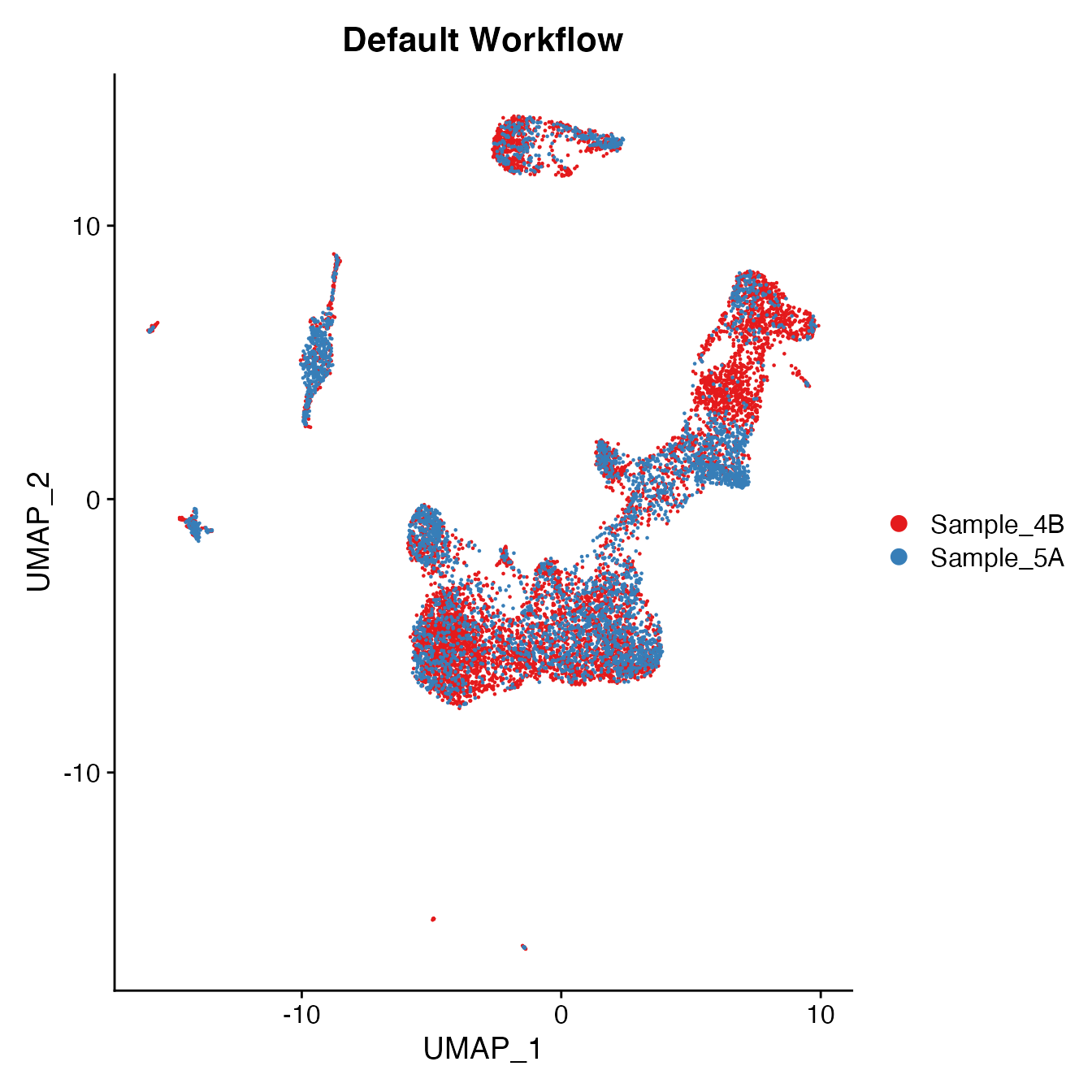
Measuring sample-UMAP integration (generating an iLISI score)
GetiLISI(object = PBMCs, nSamples = 2)## [1] 0.2949505Cell Typing of joint PBMCs object for CMS
Convert cluster classifications to cell type classifications
# For complete cell classification workflow see our vignette "Biaxial Gating of a Single Sample"
# More details can be found in figure S3 of our manuscript "Data Matrix Normalization and Merging Strategies Minimize Batch-specific Systemic Variation in scRNA-Seq Data."
UMAPPlot(PBMCs, cols = colors.use, pt.size = 2,
group.by = "seurat_clusters", label = T)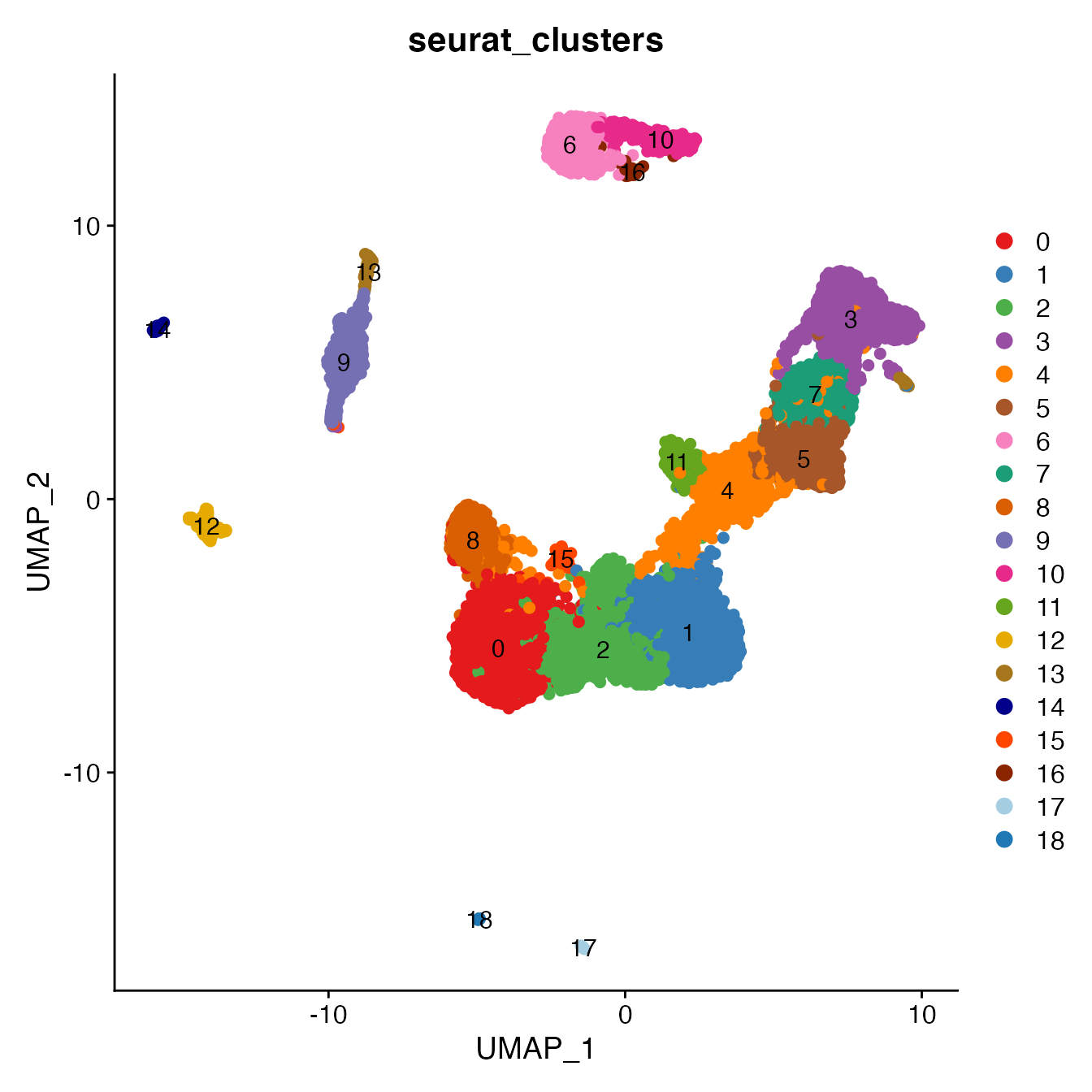
# B_Naive = 6
# B_Memory = 10, 16
# T_CD4 = 0, 1, 2, 15, 18
# TReg not a unique cluster (contained within cluster 2)
# T_CD8 = 8, 11
# NK_T = 4, 5
# NK = 3, 7
# NK_CD56Hi not a unique cluster (contained within cluster 2)
# Monocyte_Classical = 9
# Monocyte_NonClassical = 12
# Dendritic_Cells = 13, 14
# HSPCs = 17
Idents(PBMCs) <- PBMCs[["seurat_clusters"]]
Idents(PBMCs) <- plyr::mapvalues(Idents(PBMCs), from = c(6, 10, 16,
0, 1, 2, 15, 18,
8, 11, 4, 5,
3, 7, 9, 12,
13, 14, 17),
to = c('B_Naive', 'B_Memory', 'B_Memory',
'T_CD4', 'T_CD4', 'T_CD4', 'T_CD4', 'T_CD4',
'T_CD8', 'T_CD8', 'NK_T', 'NK_T',
'NK', 'NK', 'Monocyte_Classical', 'Monocyte_NonClassical',
'Dendritic_Cells', 'Dendritic_Cells', 'HSPCs'))
Idents(PBMCs) <- factor(Idents(PBMCs),
levels = c("B_Naive", "B_Memory", "T_CD4", "TReg",
"T_CD8", "NK_T", "NK", "NK_CD56Hi",
"Monocyte_Classical", "Monocyte_NonClassical",
"Dendritic_Cells", "HSPCs", "Cycling_Cells"))
PBMCs[["Cell_Type"]] <- Idents(PBMCs)
UMAPPlot(PBMCs, cols = colors.use, label = F) + ggtitle("Cell Type Classifications")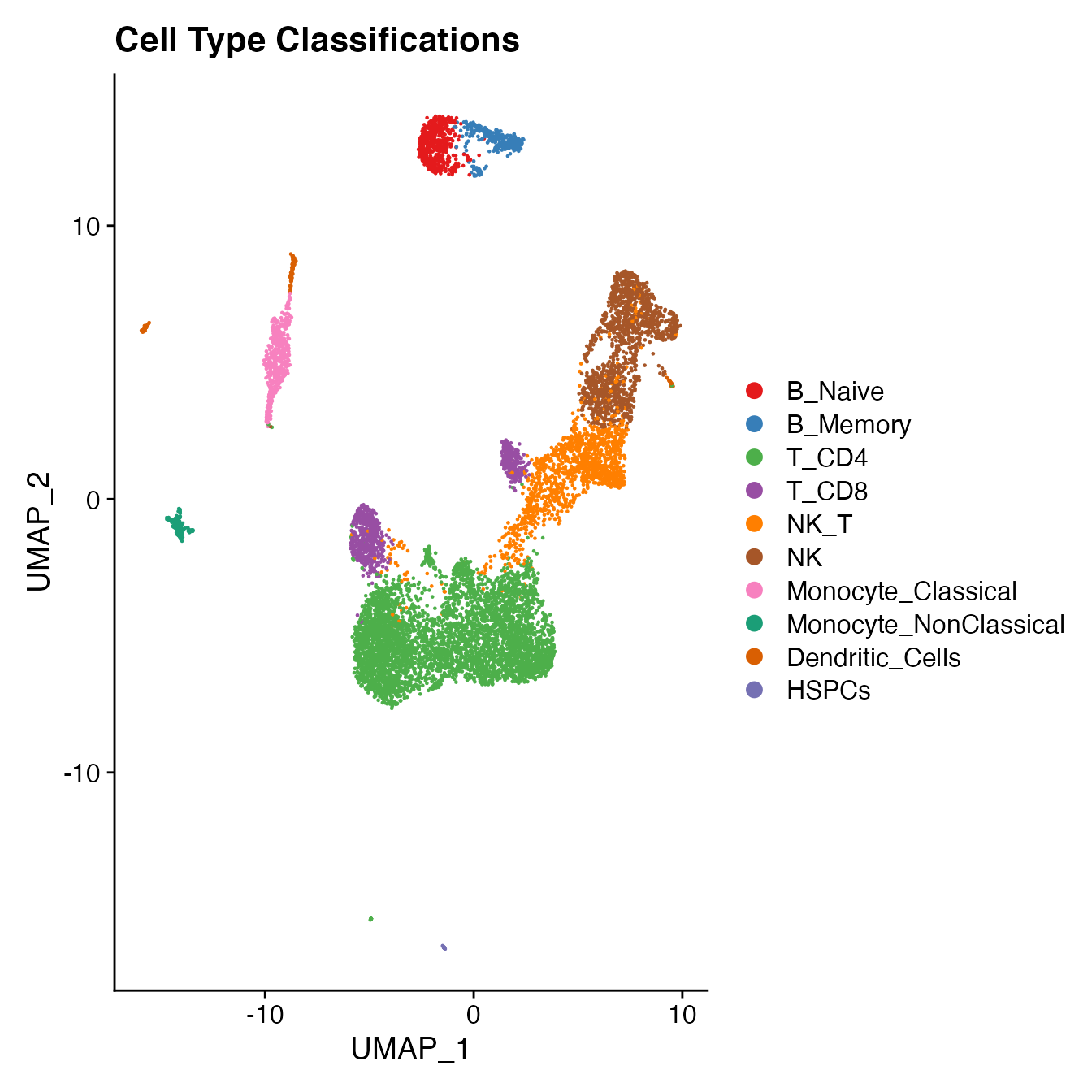
Compare single-sample workflow cell type classifications to joint classifications (generating a CMS)
# PBMC Sample 4-B
data(package = 'BatchNorm', PBMC4B_Single_ID)
S4Bcms <- GetCMS(object = PBMCs,
sample.ID = "Sample_4B",
reference.ID = PBMC4B_Single_ID)
# PBMC Sample 5-A
data(package = 'BatchNorm', PBMC5A_Single_ID)
S5Acms <- GetCMS(object = PBMCs,
sample.ID = "Sample_5A",
reference.ID = PBMC5A_Single_ID)
# Average CMS
mean(c(S4Bcms, S5Acms))## [1] 0.06950476Same Sequencing Pool (“sequenced together”)
Processing the Seurat Objects 4-A & 5-A
Data filtering & normalization
library(BatchNorm)
# Import unfiltered Seurat PBMC4 object & extract "4A" (included with 'BatchNorm' package)
data(package = 'BatchNorm', PBMC4)
Idents(PBMC4) <- PBMC4[["orig.ident"]]
PBMC4A <- subset(PBMC4, idents = "Sample_4A")
# Import unfiltered Seurat PBMC5 object & extract "5A" (included with 'BatchNorm' package)
data(package = 'BatchNorm', PBMC5)
Idents(PBMC5) <- PBMC5[["orig.ident"]]
PBMC5A <- subset(PBMC5, idents = "Sample_5A")
# Merge "sequenced together" samples into a single object and remove unnecessry objects from the workspace
PBMCs <- merge(PBMC4A, y = PBMC5A)
rm(list = c('PBMC4', 'PBMC5', 'PBMC4A', 'PBMC5A'))
# Run "standard" Seurat workflow:
# Including filtering by mitochondrial percentage (+5 SD)
# Including data normalization, variable gene selection and gene scaling (performed on all samples together)
PBMCs <- PBMCs %>%
MitoFilter() %>%
NormalizeData(normalization.method = "LogNormalize", assay = "RNA", scale.factor = 10000) %>%
NormalizeData(verbose = FALSE, assay = "ADT", normalization.method = "CLR") %>%
FindVariableFeatures(selection.method = "vst", nfeatures = 2000) %>%
ScaleData() %>%
RunPCA(npcs = 30)Selecting the appropriate number of Principal Components for UMAP reduction
## Identify correct numbers of PCs
## (Takes up to 5 minutes. Not run while rendering vignette for time)
# PBMCs.pca.test <- TestPCA(PBMCs)
# PBMCs.pca.test[, 1:20]
## 14 PCs with z > 1
## Proceed with 14 PCs for dimensional reduction & clustering
## Visualize PCs plotted by standard deviation:
ElbowPlot(PBMCs)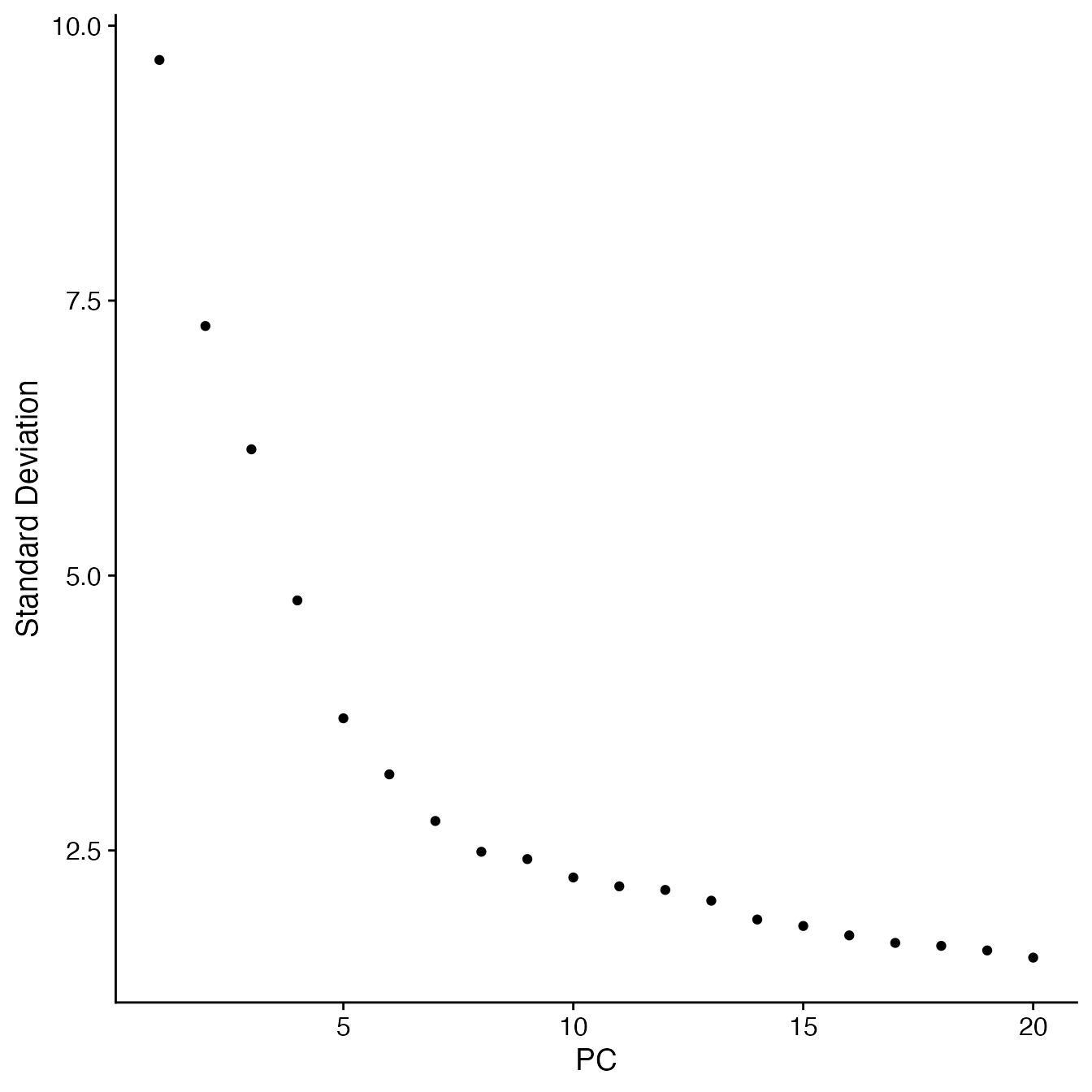
Generating a UMAP and clusters
PBMCs <- PBMCs %>%
RunUMAP(reduction = "pca", dims = 1:14) %>%
FindNeighbors(reduction = "pca", dims = 1:14) %>%
FindClusters(resolution = .8)## Modularity Optimizer version 1.3.0 by Ludo Waltman and Nees Jan van Eck
##
## Number of nodes: 10101
## Number of edges: 353466
##
## Running Louvain algorithm...
## Maximum modularity in 10 random starts: 0.8759
## Number of communities: 21
## Elapsed time: 1 seconds
UMAPPlot(PBMCs, cols = colors.use, group.by = "orig.ident") + ggtitle("Default Workflow")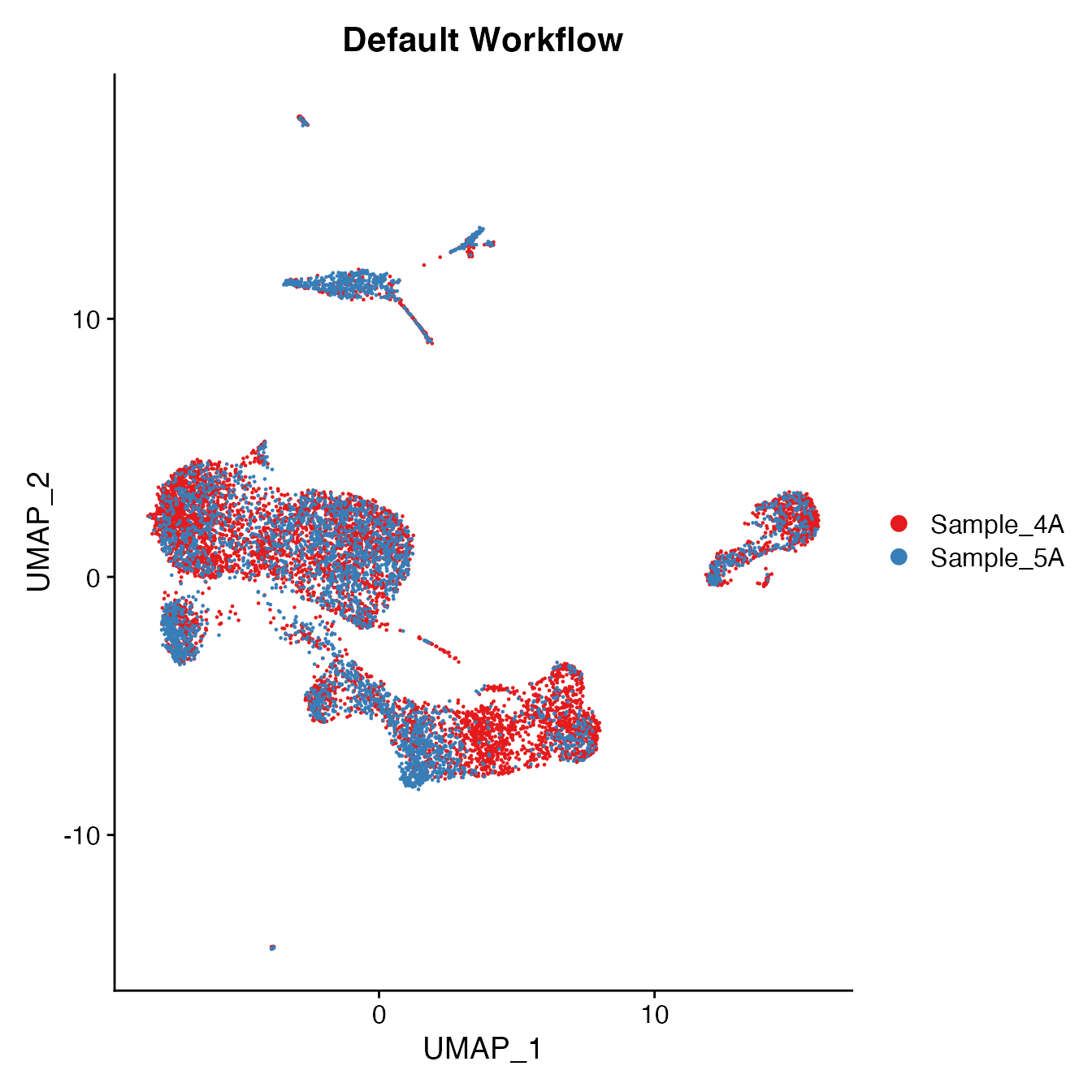
Measuring sample-UMAP integration (generating an iLISI score)
GetiLISI(object = PBMCs, nSamples = 2)## [1] 0.2792723Cell Typing of joint PBMCs object for CMS
Convert cluster classifications to cell type classifications
# For complete cell classification workflow see our vignette "Biaxial Gating of a Single Sample"
# More details can be found in figure S3 of our manuscript "Data Matrix Normalization and Merging Strategies Minimize Batch-specific Systemic Variation in scRNA-Seq Data."
UMAPPlot(PBMCs, cols = colors.use, pt.size = 2,
group.by = "seurat_clusters", label = T)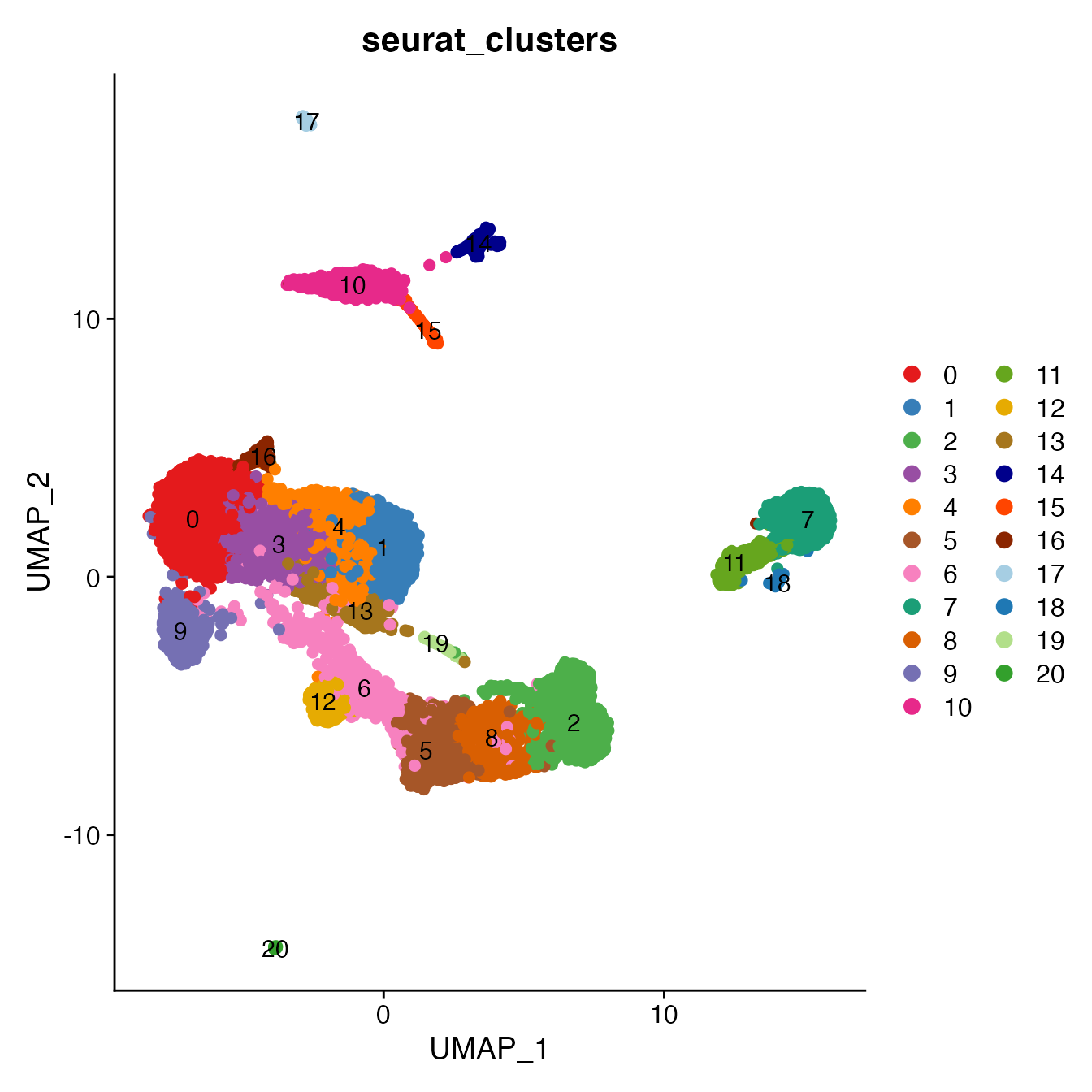
# B_Naive = 7
# B_Memory = 11, 18
# T_CD4 = 0, 1, 3, 4, 16
# TReg = 13, 19
# T_CD8 = 9, 12
# NK_T = 5, 6
# NK = 2, 8
# NK_CD56Hi not a unique cluster (contained within cluster 2)
# Monocyte_Classical = 10
# Monocyte_NonClassical = 14
# Dendritic_Cells = 15, 17
# HSPCs = 20
Idents(PBMCs) <- PBMCs[["seurat_clusters"]]
Idents(PBMCs) <- plyr::mapvalues(Idents(PBMCs), from = c(7, 11, 18, 0, 1, 3, 4, 16,
13, 19, 9, 12, 5, 6,
2, 8, 10, 14,
15, 17, 20),
to = c('B_Naive', 'B_Memory', 'B_Memory', 'T_CD4', 'T_CD4', 'T_CD4', 'T_CD4', 'T_CD4',
'TReg', 'TReg', 'T_CD8', 'T_CD8', 'NK_T', 'NK_T',
'NK', 'NK', 'Monocyte_Classical', 'Monocyte_NonClassical',
'Dendritic_Cells', 'Dendritic_Cells', 'HSPCs'))
Idents(PBMCs) <- factor(Idents(PBMCs),
levels = c("B_Naive", "B_Memory", "T_CD4", "TReg",
"T_CD8", "NK_T", "NK", "NK_CD56Hi",
"Monocyte_Classical", "Monocyte_NonClassical",
"Dendritic_Cells", "HSPCs", "Cycling_Cells"))
PBMCs[["Cell_Type"]] <- Idents(PBMCs)
UMAPPlot(PBMCs, cols = colors.use, label = F) + ggtitle("Cell Type Classifications")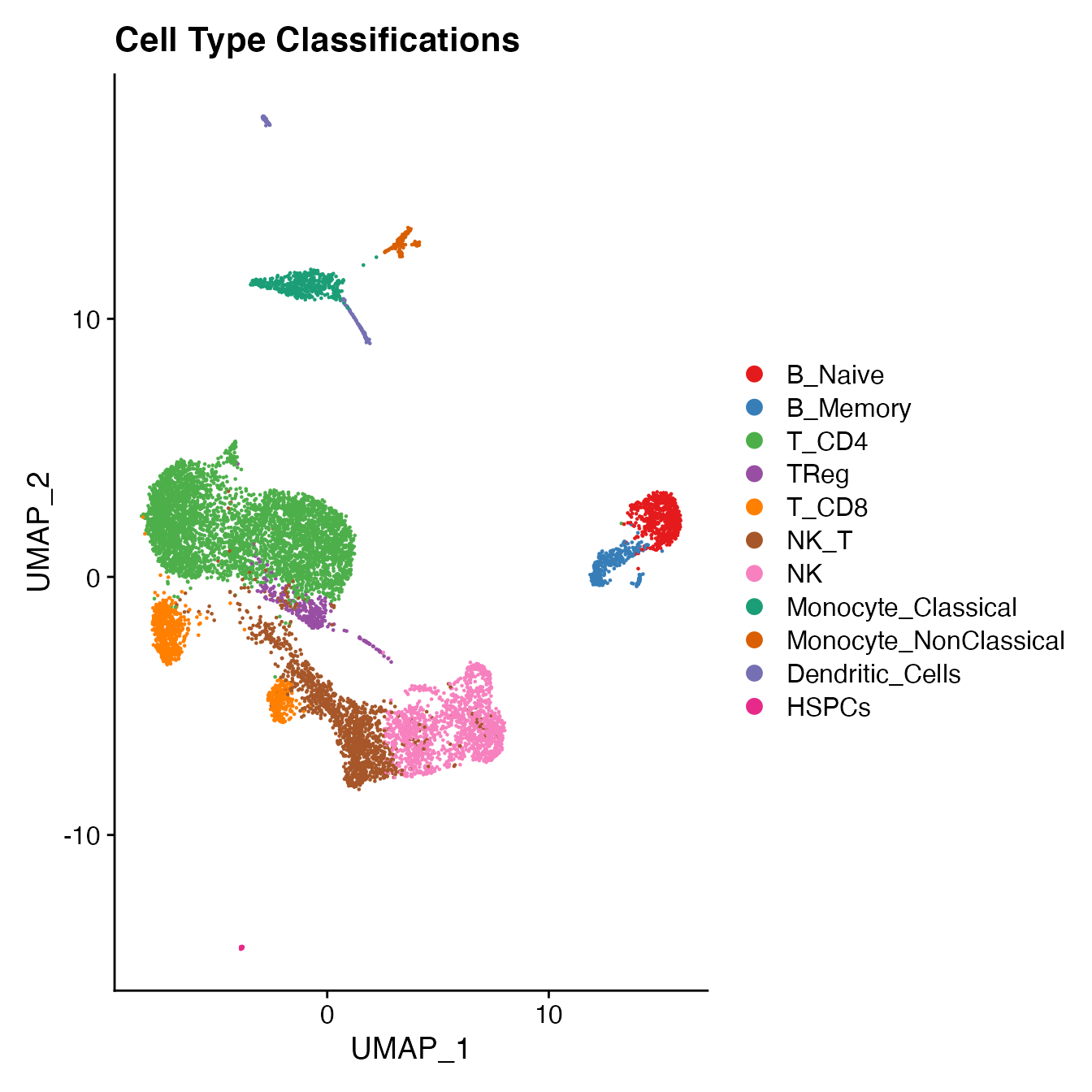
Compare single-sample workflow cell type classifications to joint classifications (generating a CMS)
# PBMC Sample 4-A
data(package = 'BatchNorm', PBMC4A_Single_ID)
S4Acms <- GetCMS(object = PBMCs,
sample.ID = "Sample_4A",
reference.ID = PBMC4A_Single_ID)
# PBMC Sample 5-A
data(package = 'BatchNorm', PBMC5A_Single_ID)
S5Acms <- GetCMS(object = PBMCs,
sample.ID = "Sample_5A",
reference.ID = PBMC5A_Single_ID)
# Average CMS
mean(c(S4Acms, S5Acms))## [1] 0.06985096